Almost Living Cell-Free Expression
ALiCE® is a scalable eukaryotic cell-free protein expression system capable of producing even the most complex proteins in under 48 hours. Our proprietary cell-free lysate contains all of the machinery necessary to implement eukaryotic post-translational modifications, without specific optimizations.
Furthermore, the ALiCE® system consistently produces protein yields of up to a minumum of an industry-leading 2 grams per liter.
Without the constraints imposed by living systems, our cell-free system has the advantage of being able to produce all proteins, even toxic ones.
-
> 2 Grams per Liter Protein Yield
-
Complex Post-Translational Modifications
-
Protein in Under 48 Hours
-
Scalable Reactions to 10 L
What Is ALiCE®?
ALiCE® is a plant-based cell-free lysate derived from Nicotiana. tabacum c.v. BY-2 root cells. ALiCE® stands for Almost Living Cell-Free Expression. The lysate is prepared by removing the cell wall and other cell components that are not necessary for protein production, leaving only the the protein production machinery and mitochondria which power the reaction. During our lysate finalization steps, the additional components necessary for protein production (amino acids, co-factors, etc.) are added to produce something that functions just like a cell, but without the constraints of a living system – all that’s needed is the DNA encoding the protein of interest!
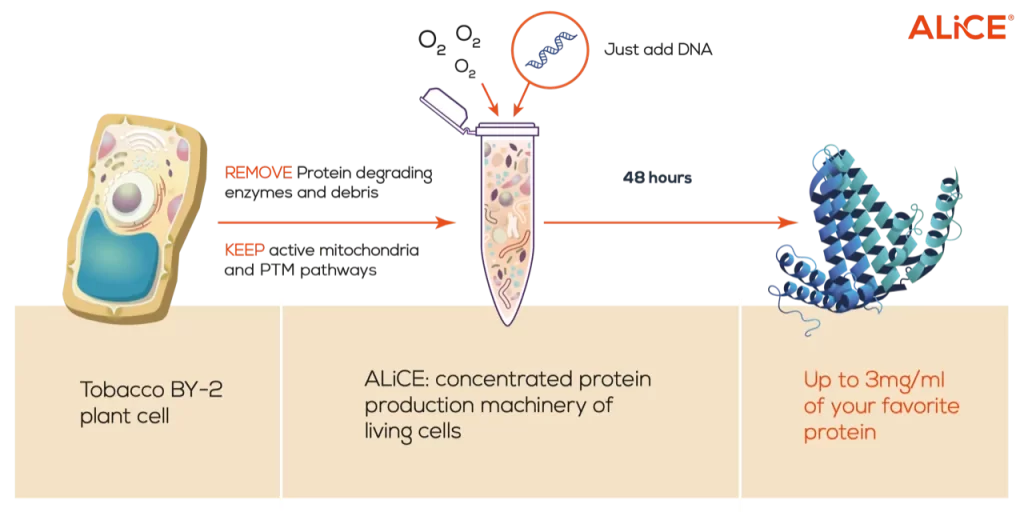
ALiCE® Has A Proven Track Record
Our ALiCE® lysate can produce all classes of protein, including those that are typically difficult to express. Because our lysate contains native microsomes derived from the endoplasmic reticulum and Golgi, it is able to introduce complex eukaryotic post-translational modifications including N-glycosylation and disulfide bonds.
Published examples of proteins expressed in ALiCE® include:
- Membrane proteins
- Antibodies
- Protein complexes (e.g. VLPs)
- Growth factors
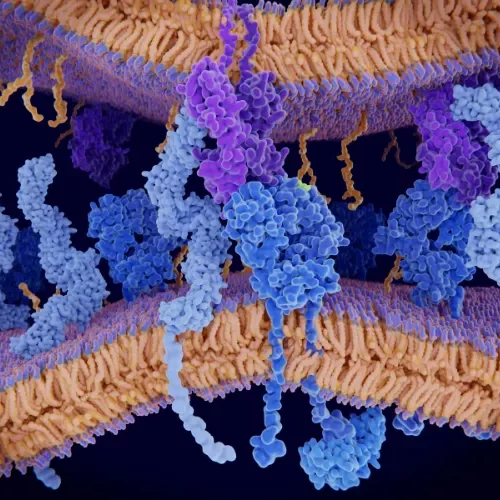
Membrane Proteins
A eukaryotic cell-free platform for reliable expression of functional membrane proteins
Due to their cell surface accessibility and importance in cellular regulation, membrane proteins are significant drug targets. However, they are challenging to obtain due to their aggregation-prone nature, resulting in low expression and purification yields.
ALiCE® in action:
- Two human G-coupled receptors (GPCRs) – CB2 and ADRB2 – were produced using the ALiCE® cell-free expression system in a 24 hour reaction.
- It was possible to assess GPCR functionality within the native microsome environment without purification.
- The activity profile of CB2 produced using ALiCE® was comparable to the protein expressed using a cell-based system.
Conclusion
ALiCE® is particularly suitable for membrane protein expression because it contains native microsomes from the endoplasmic reticulum and Golgi. These microsomes act as a scaffold for the insertion of membrane proteins. Unlike cell-based systems, ALiCE® does not require cell lysis for downstream processing.
Antibodies
Expression of a functional therapeutic antibody in the ALiCE cell-free protein expression system
Monoclonal antibodies (mAbs) and their related therapeutic antibody variants are essential components in the field of medical treatment, particularly in the management of cancer and autoimmune diseases. These antibodies are engineered to specifically target and interact with particular molecules or cells in the body, thereby offering a highly targeted and precise approach to therapy.
ALiCE® in action:
- Adalimumab (aka Humira®), a complex therapeutic antibody with 12 intra- and 4 intermolecular disulfide bonds and 2 glycosylation sites, was successfully produced using the ALiCE® system.
- Heavy and light chains were simultaneously expressed in a one-pot reaction, resulting in fully assembled tetrametic antibody in 24 hours.
- Adalimumab produced in ALiCE® showed similar antigen binding to CHO-produced antibody.
Conclusion
The ALiCE® cell-free expression system can significantly expedite the process of antibody screening.

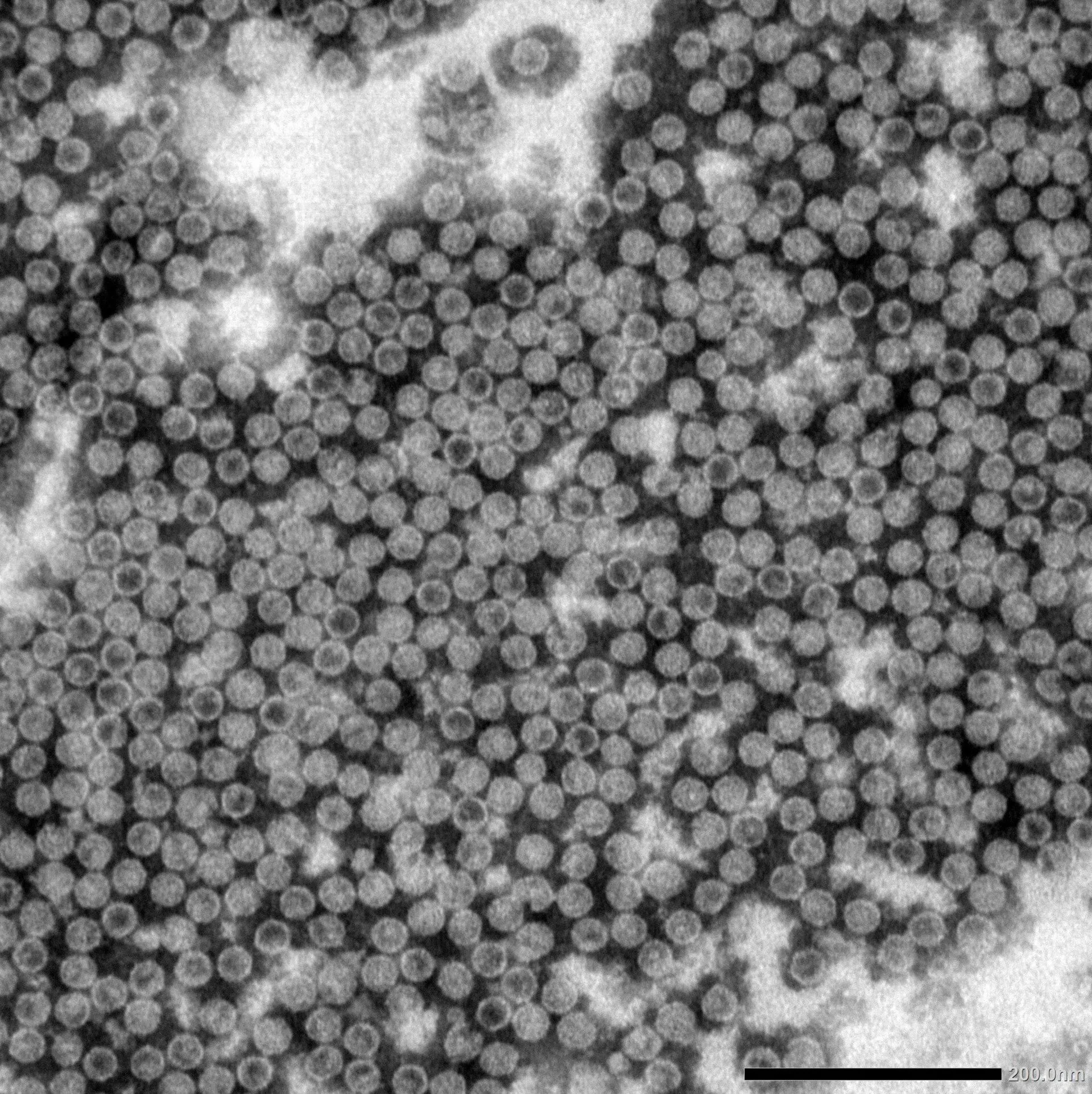
Virus-Like Particles
Expressing Virus-Like Particles using the Cell-Free Protein Expression System
Virus-Like Particles (VLPs) are multi-subunit proteins that are considered a promising approach for vaccines because they can elicit robust and long-lasting immune responses resembling those generated by viruses. Importantly, they pose fewer safety concerns as they lack the genetic material and machinery necessary for viral replication.
ALiCE® in action:
- Using the ALiCE® expression system, high quality, fully assembled Hepatitis B Core (HBc) Carrier VLPs were produced.
- VLPs produced by ALiCE® are capable of inducing the expected immune response.
Conclusion
The ALiCE® cell-free expression system can significantly expedite the process of antibody screening.
The first scalable eukaryotic expression system
Cell-free protein expression systems (CFPS) offer many benefits over cell-based alternatives, including shorter protein production time (hours vs. months), and the ability to produce proteins at levels usually toxic to living cells. However, the widespread adoption of CFPS in industrial protein manufacture has been hindered by a lack of systems that can reliably operate at scale.
In a world first, we have successfully scaled ALiCE® to produce up to a minimum of 2 mg/mL protein in reaction volumes of up to 10 liters, without requiring extensive process development and optimization. This opens the door to applications of our technology beyond research and development.
Read about the benefits of cell-free compared to cell-based expression systems
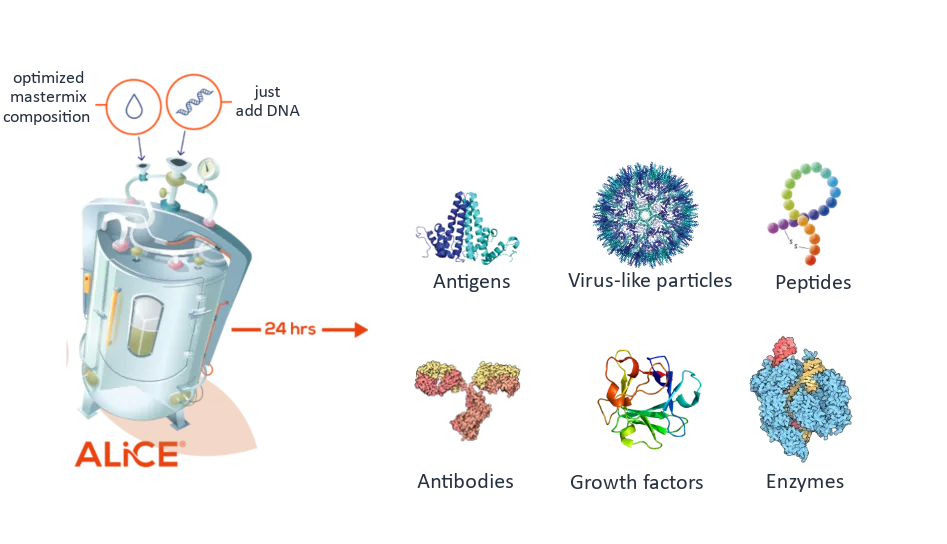
How does ALiCE® work?
Producing eukaryotic proteins with yields of up to a minimum of 2 g/liter couldn’t be easier with ALiCE®: simply add plasmid DNA containing your cloned gene-of-interest, incubate for 24-48 hours and retrieve your protein.
Download our White Paper and discover the potential of ALiCE® to redefine protein production
ALiCE® as a Product
Our ALiCE® lysate is currently available as both a protein expression kit for small-scale expression experiments and as a protein service. If you are interested in purchasing ALiCE® in larger volumes for protein manufacture at scale, please contact our business development team.

ALiCE® for Research
ALiCE® is available as a cell-free protein expression kit in three different sizes to suit your needs.

ALiCE® as a Service
We offer an end-to-end protein service from cloning to expression and purification.

ALiCE® for Scale-Up
Interested in producing your protein of interest in large amounts? Contact our Sales team.
