The First Fully Scalable Eukaryotic Cell-Free Expression Platform
From screening campaign to characterization and developability assessment-scale protein production in a matter of days – without the need for cell line development or process optimization. ALiCE®, our pioneering scalable eukaryotic cell-free protein synthesis platform, makes this dream a tangible reality, accelerating protein exploration across a spectrum of applications in basic research to biopharma development.

Challenging the Protein Expression Status Quo
For decades, cell-free protein synthesis has been renowned for its ability to rapidly express proteins resistant to production in cell-based systems. Yet, the challenge of scaling eukaryotic systems, essential for producing complex proteins, has hindered widespread adoption and limited scientists leveraging the full power of this technology. ALiCE® is different: Our team of innovators have developed ALiCE® to work as effectively in microliter volumes as in liter-scale reactions.
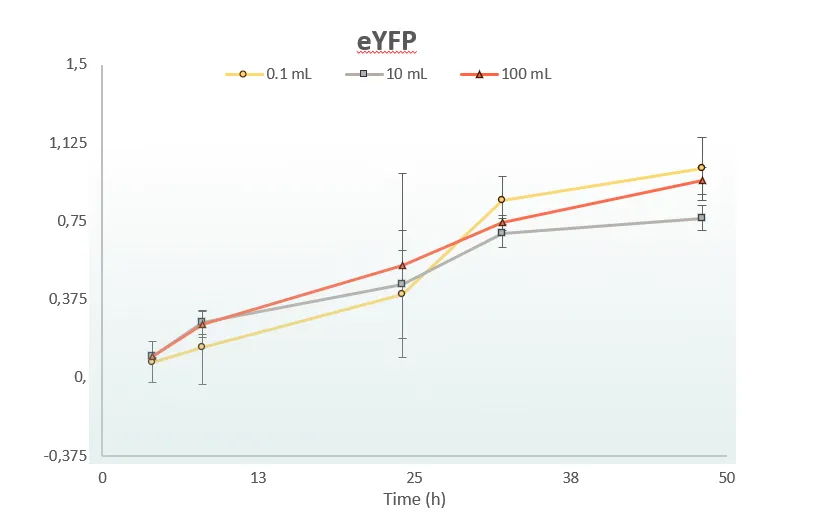
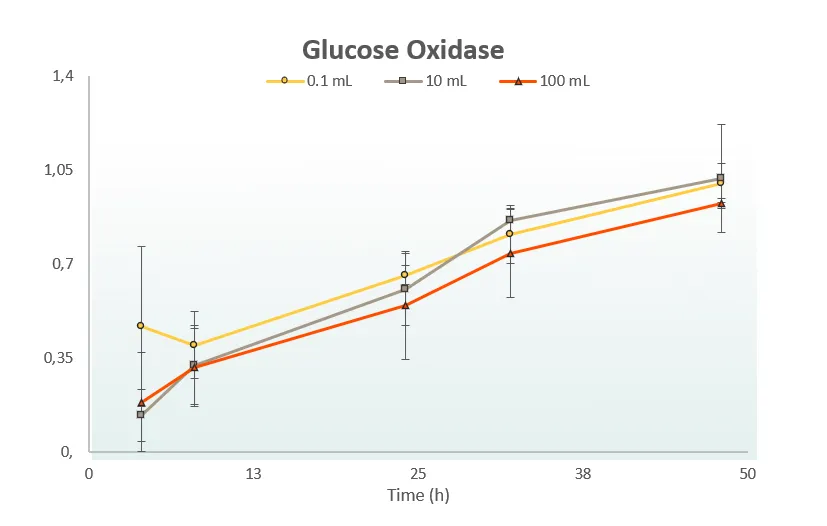
One Lysate – Any Scale – Just Add DNA
ALiCE® has been developed for optimal ease of use across scales. From microliter reactions in microtiter plates, through milliliter volumes utilizing conical flaks, to liter-scale reactions in bioreactors, our protocols are optimized for use with standardly available lab equipment. Whatever the scale, it’s the same simple protocol – just add the DNA sequence for your protein and let ALiCE® make your protein. Contact our team to learn how ALiCE® can work with your established equipment.

Interested in Using ALiCE® for Scaled Protein Production?
Contact Us
A Transferable Process on Track for GMP
We’ve made significant strides in expanding access to the power of ALiCE®, but our journey is far from over. Our objective is to ensure that ALiCE® is accessible for protein production at any scale, catering not only to research-grade proteins but also to clinical trials and biopharma manufacturing. With a robust ALiCE® manufacturing process in place, we’re forging a clear path towards a fully transferable process and compliance with GMP standards.
Want to learn more? Contact Us

