Better preparedness saves lives – this is one of the most painful takeaways from the coronavirus pandemic. Despite the unprecedented timelines achieved for vaccine development, a feat that undoubtedly deserves much praise, we still wonder how many lives could have been saved if effective vaccines were made available faster. Is better preparedness what we need to avert the next pandemic? In this article, we introduce a cell-free protein expression (CFPE)-based pandemic-preparedness vaccination platform that could be rapidly engineered to produce vaccines against different pathogens at scale.
Developing a vaccine in 100 days? A mission not impossible
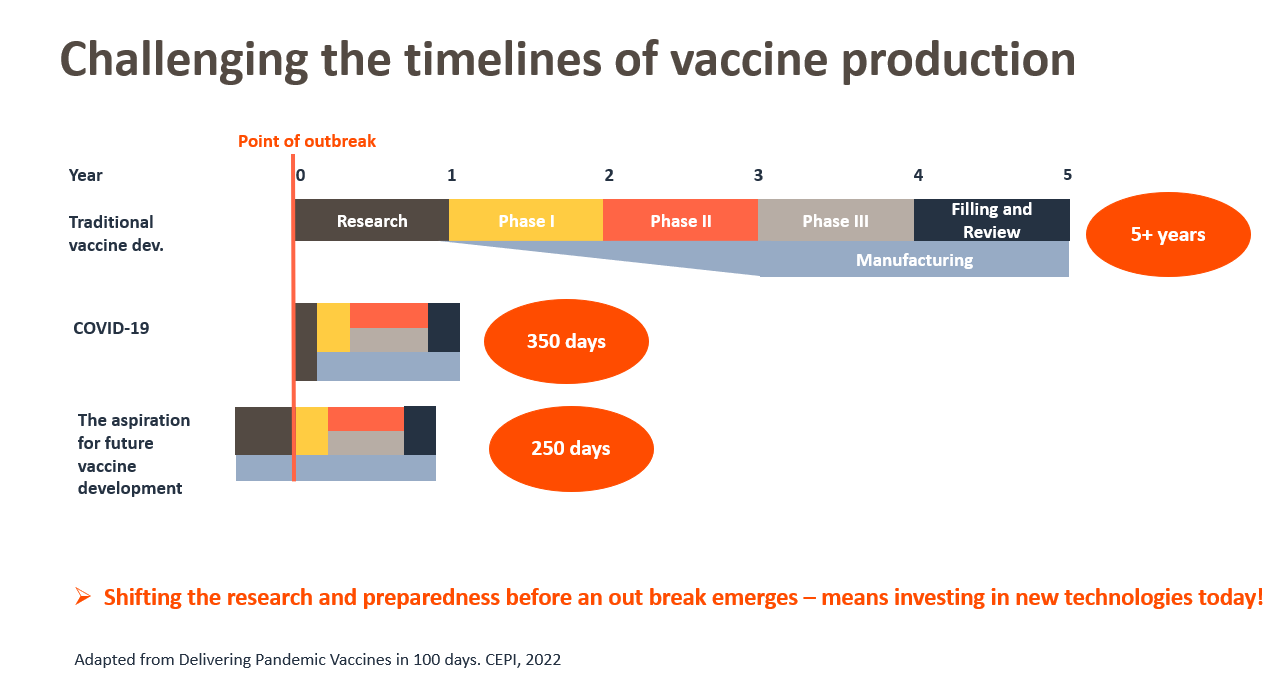
The coronavirus pandemic was a test to humanity in many ways. How did we fare? The timeline from sequence to emergency use authorization by a stringent regulatory authority or Emergency Use Listing (EUL) by the World Health Organization (WHO), ranged from 326 to 706 days (1), setting new records for time to approval of a new vaccine. But what if we can bring this down to 100 days?
CEPI, a global coalition with the mission to accelerate vaccine development in response to epidemic and pandemic threats, along with the UK Government, recently hosted the Global Pandemic Preparedness Summit to explore this possibility. The Summit report highlights the importance "to shift beyond the current vaccine development paradigm towards preparedness" to achieve the aspirational 100-day timeline (1).
Getting novel rapid response vaccine development platforms ready for deployment
One of the key pillars of the CEPI 100-day strategy is to get scalable pandemic-preparedness vaccination platforms ready for deployment. A multi-prong approach with different pandemic-preparedness vaccination platforms might be the best strategy to tackle not just rapid vaccine development but also worldwide distribution.
Current vaccines can be broadly classified into four types based on how they are developed (2):
- Conventional whole virus vaccines, including live-attenuated and inactivated vaccines, are the oldest and most well-established vaccine types. However, their manufacturing is time-consuming and requires dedicated higher biosafety level production facilities and extensive safety evaluations.
- Protein-based vaccines, including protein subunit and virus-like particle (VLP) vaccines, lack genetic materials and contain only viral proteins. Due to the lack of an intact virus, these vaccine types cannot cause infection and, therefore, are safer and easier to produce.
- Viral vector vaccines, which include non-replicating and replicating types, use non-pathogenic vectors to deliver antigen-coding DNA fragments to host cells. Host cells then produce the antigen using their own protein-synthesis machinery. These vaccines don't need to be transported or stored at extremely low temperatures and can be produced rapidly on a large scale. However, prior immunity to the vector may restrict its capacity to transfer genetic material to host cells, decreasing the vaccine's efficacy.
- Nucleic acid vaccines, which include DNA and RNA vaccines, also leverage the host cell's protein synthesis machinery to generate antigens. DNA vaccines use plasmid DNA containing a mammalian expression promoter and a transgene encoding the protein antigen. mRNA vaccines contain mRNA encapsulated in lipid nanoparticles (LNPs) for cellular delivery. While DNA vaccines are stable at room temperature, several mRNA vaccines have strict cold chain requirements. Rapid scaling is one of the main benefits of these platforms.
Download our latest application note, and learn how fully-formed protein complexes were produced using ALiCE
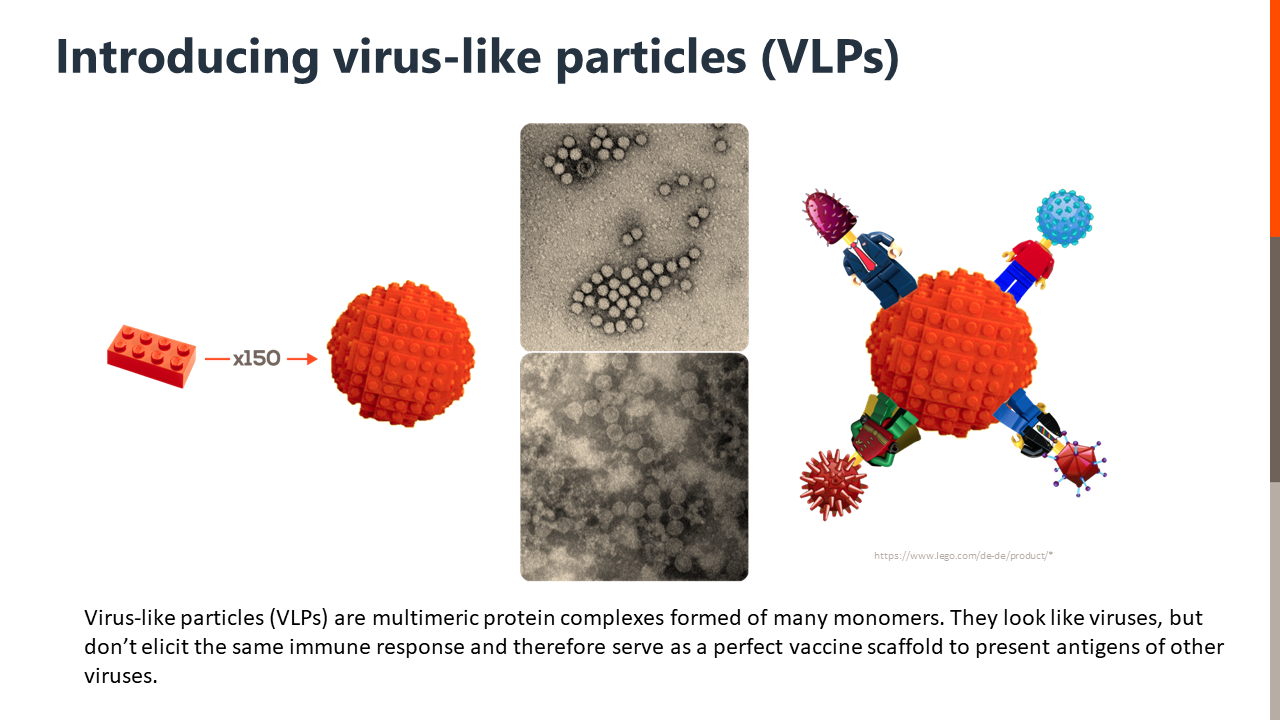
VLPs – a promising addition to the vaccine development toolbox
Due to their semblance to viruses, VLP-based vaccines stimulate solid and long-lasting immune responses while posing fewer safety issues as they lack the genetic material and molecular machinery necessary for viral reproduction (3).
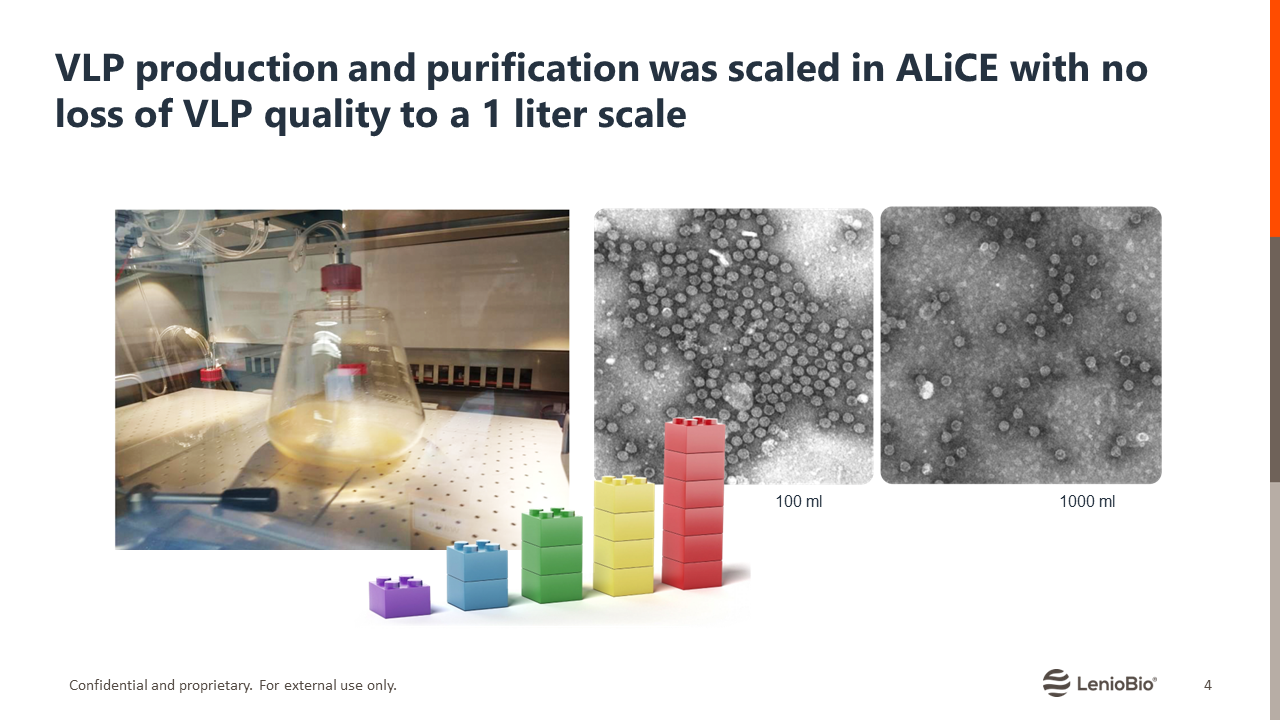
A recent article published in Frontiers in Immunology describes using a scalable cell-free protein expression system – ALiCE® – for rapid screening and production of Hepatitis B core (HBc) carrier VLPs. ALiCE® is a cell-free lysate derived from Nicotiana tabacum c.v. BY-2 cells containing all of the machinery necessary to implement eukaryotic post-translational modifications without specific optimizations. Using ALiCE, the researchers expressed and screened an array of HBc-VLP constructs within 48 hours. Furthermore, they showed that the reaction is scalable up to 1 liter without affecting VLP assembly or yield and demonstrated the immunogenicity of the CFPE-produced VLPs using in vitro assays (4).
Slide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButton
Conclusion
Cell-free expression systems are redefining biomanufacturing. This study establishes LenioBio GmbH's proprietary cell-free system ALiCE® as a potential pandemic-preparedness vaccination platform for rapid production and screening of VLP-based candidates in response to emerging pandemic threats. Its rapid synthesis capabilities, together with its immunogenicity, scalability, and flexibility, make ALiCE® a promising platform to meet ambitious missions like the 100-day vaccine development challenge.
Do you have a candidate VLP vaccine for co-development? Contact our business development team.
References
- CEPI 100 days report. 2022. Delivering pandemic vaccines in 100 days: what will it take?Available at: https://100days.cepi.net/wp-content/uploads/2022/12/CEPI-100-Days-Report-Digital-Version_29-11-22.pdf [Accessed February 2023].
- Li Y, Tenchov R, Smoot J, Liu C, Watkins S, Zhou Q. A comprehensive review of the global efforts on COVID-19 vaccine development. ACS Cent Sci. 2021; 7(4):512-533.
- Mohsen MO, Zha L, Cabral-Miranda G, Bachmann MF. Major findings and recent advances in virus-like particle (VLP)-based vaccines. Semin Immunol. 2017; 34:123–32.
- Armero-Gimenez J, Wilbers R, Schots A, Williams C, Finnern R. Rapid screening and scaled manufacture of immunogenic virus-like particles in a tobacco BY-2 cell-free protein synthesis system. Front. Immunol. 2023.